How to Determine Which Wave Function to Use
De Broglie relation we should use a periodic function with wavelength hp. The Wave Function PDF 4 Expectations Momentum and Uncertainty PDF 5 Operators and the Schrödinger Equation PDF 6 Time Evolution and the Schrödinger Equation PDF 7 More on Energy Eigenstates PDF 8 Quantum Harmonic Oscillator.

Ask Ethan What S The Difference Between A Fermion And A Boson Wave Function Quantum Mechanics Quantum Physics
The solution is only good to a multiplicative constant so you add such a constant A nl which turns out to depend.

. However the wave function above tells us nothing about where the particle is to be found in space. The Schrodinger Equation. Generally we will establish the energy graph first.
YxtAcosomega tbeta xphi in this equation omega t and beta x symbols of the coefficient are same ie or -- then the wave is negative direction travelling wave. It is expressed as ψ x y z t a ib and the complex conjugate of the wave function is expressed as ψ x y z t a ib. You would use the negative sign if the wave is moving to the right and the positive sign if the wave was moving to the left.
The energy of an individual photon depends only on the frequency of light ϵphoton hf so E 2 is proportional to the number of photons. The wave amplitude for each position is the square root of 16 written 16. ω ω 1 ω 2 ω m continuous variables not necessarily dimensionless These quantum numbers index the components of the state vector.
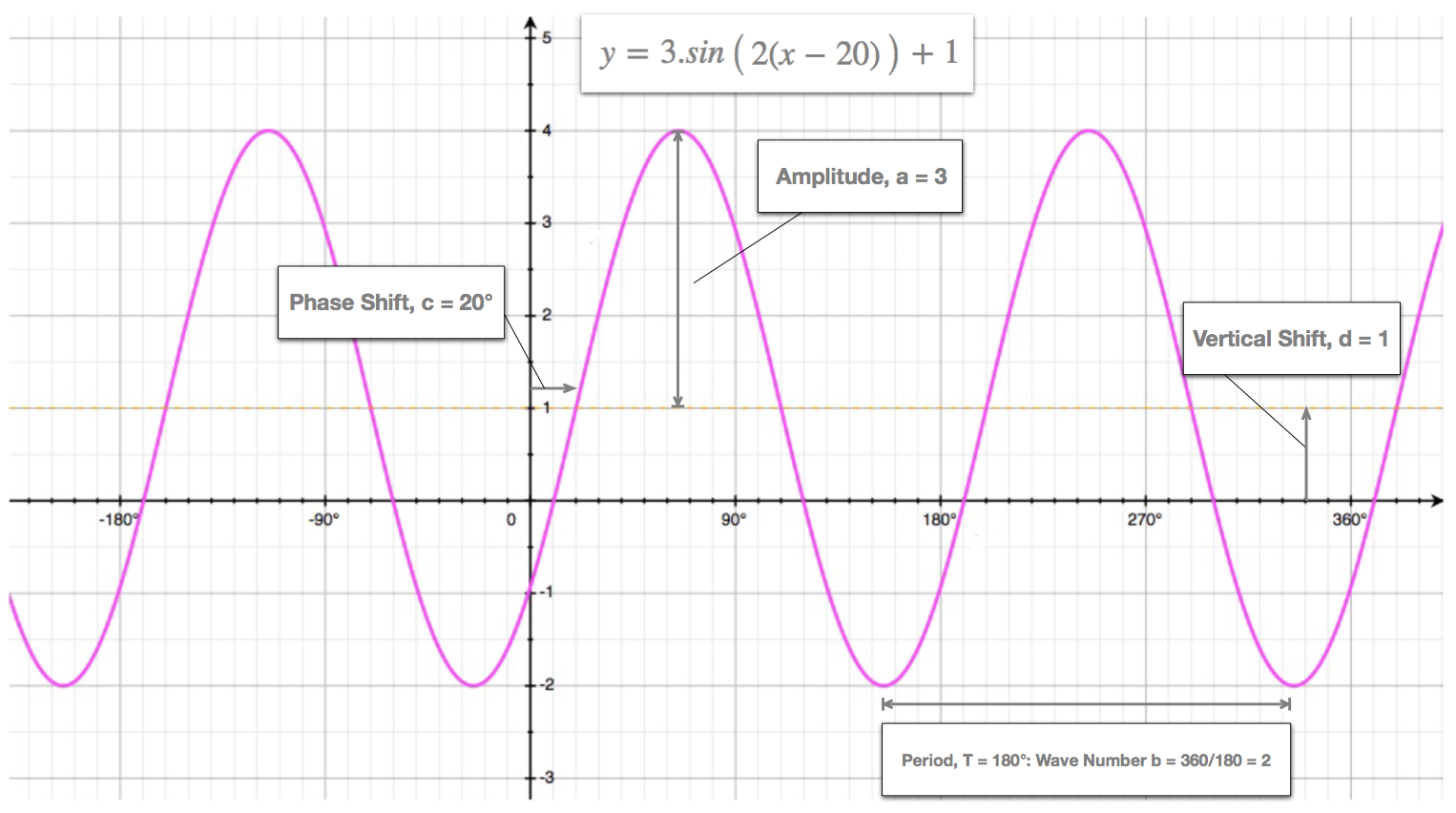
B is known as the wave number also called the angular frequency. We can make this statement because this wave function is more or less the same everywhere. And y a.
Cosbx c d. C is known as the phase shift. To help you draw wave functions use the Wave Function Sketcher program.
YxtAcosomega tbeta xphi in this equation omega t and beta x symbols of the coefficient are alternative ie - or - then the wave is positive direction travelling wave. However the Schrodinger equation is a wave equation for the wave function of the particle in question and so the use of the equation to. To figure out how to normalize your wavefunction you need to know what the integral of a Gaussian function is where a Gaussian function is any function of the form.
Heres what the integral in this equation equals. Sinbx c d Where. In this case for each position it happens to be a.
This guarantees that the wave function returns a single value for the probability for any state. A long wavelength would correspond to a small momentum and a short wavelength would correspond to a large momentum. α α 1 α 2 α n dimensionless discrete quantum numbers.
Using the Wave Function. Our first situation will be the one described in Figure 1. Read the problem and identify if any variables are given.
A is known as the amplitude. This video discusses the physical meaning of wave function normalization and provides examples of how to normalize a wave function. Substituting for gives you the following.
There are 4 main conditions that we need for a function to be a valid wave function. The pro-gram has a window for drawing wave functions and another for the energies. Therefore heres the normalized wave equation with the value of A plugged in.
Ψα ω t a component of the vector Ψ called the wave function of the system. This will be automatically true for any valid mathematical function since this is a condition on functions from mathematical analysis. Sawtooth Function Wave The sawtooth function named after its saw-like appearance is a relatively simple discontinuous function defined as f t t for the initial period from -π to π in the above image.
The wave function must be single valued. This periodic function then repeats as shown by the first and last lines on the above image. A clue to the physical meaning of the wave function is provided by the two-slit interference of monochromatic light.
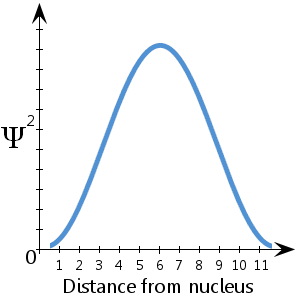
See also Electromagnetic Waves and Interference The wave function of a light wave is given by Ext and its energy density is given by where E is the electric field. Using the following wave function graph as seem below determine the most likely position of the electron. This graph is based on the physical situation.
According to the Born rule we square the amplitude and get the probability that the electron will be detected in each position. The additional periods are defined. A true sinusoidal function would repeat.
More all α are in an n-dimensional set A A 1 A 2. Practice Using a Graph of a Wavefunction to Determine the Most Likely Position of a Particle with practice problems and explanations. If your quantum physics instructor asks you to find the wave function of a hydrogen atom you can start with the radial Schrödinger equation R nl r which tells you that The preceding equation comes from solving the radial Schrödinger equation.
There is no physical meaning of wave function as it is not a quantity which can be observed. Perhaps the simplest way to approximate the shape is with the sine function and inverse sine function. We could also try to learn from the wave function the position of the particle.
In a normalized function the probability of finding the particle between adds up to 1 when you integrate over the whole square well x 0 to x a. The simplest periodic function would be a sine or a cosine which would look like this. Instead it is complex.
The wavefunction of a light wave is given by E x t and its energy density is given by E 2 where E is the electric field strength. In their most general form wave functions are defined by the equations. The Schrodinger equation is linear partial differential equation that describes the evolution of a quantum state in a similar way to Newtons laws the second law in particular in classical mechanics.
So from the previous equation Solve for A. For example the following graph uses a combination of sine and inverse sine to create the triangular waves. Properties of periodic waves Our mission is to provide a free world-class education to anyone anywhere.
The particle from the phase velocity of its wave function v 2vp.
Definition Of Wave Function Chemistry Dictionary

The Measurement Problem In Quantum Mechanics Is The Problem Of How Or Whether Wave Function Collapse Quantum Mechanics Tattoo Quantum Mechanics Wave Function

16 2 Mathematics Of Waves University Physics Volume 1

Frequency Formula Period Time Frequency Cycle Per Second Hertz Hz Amplitude Duration Periodic Time P Ultrasound Physics Physics And Mathematics College Physics

How To Calculate The Wave Speed Of A Wave When Wavelength And Frequency Chemistry Class Speed Frequencies

Pin By Randall Stebbins On Physics Quantum Mechanics Physics Physics Memes

Matter Wave Wikipedia The Free Encyclopedia Physics Problems Physics And Mathematics Physics Mechanics

Physics Page Timeline Photos Facebook Wave Function Many Worlds Interpretation Wave Equation

Physics Page Timeline Photos Facebook Wave Function Many Worlds Interpretation Wave Equation

Quantum Chemistry Breakthrough Deepmind Uses Neural Networks To Tackle Schrodinger Equation Synced Condensed Matter Physics Quantum Computer Quantum Physics
Transformed Cosine Sine Curves Wave Function

Conceptual Questions Band Theory Of Solids By Openstax Page 2 7 Jobilize Llc Theories Wave Function Conceptual

Wave Functions An Overview Sciencedirect Topics

Normalizing A Wavefunction Youtube

Wave Function For A Particle In A Potential Well Illustration By Physics Teacher Yuri Kovalenok Jurij0 Engineering Notes Physics Notes Learn Physics

Comments
Post a Comment